The Franklin Marble at the Lime Crest quarry near Sparta, New Jersey is also host to graphite crystals of exceptional quality. Besides well-formed crystals, some very unusual graphite morphologies can also be found.
Bulk samples of graphite-bearing marble were collected from a graphite-rich zone on the lower level of the quarry by John Rakovan on April 29, 1991, and generously supplied to the author for examination. Bulk material was subsequently trimmed and examined with an optical stereo microscope. A dilute solution of hydrochloric acid (HCl) was used to partially etch away the calcite in many specimens in order to expose the enclosed, protected graphite crystals. Although graphite crystals occur in the marble to over 1 cm across, the best crystals are usually less than 2 mm across. Associated minerals include crystals of phlogopite, pyrite, pyrrhotite, molybdenite, arsenopyrite, sphalerite and a few unidentified species. The identities of the pyrrhotite, pyrite, molybdenite and arsenopyrite were confirmed by the use of energy dispersive spectroscopy (EDS) capabilities of a scanning electron microscope (SEM) at Michigan Technological University's Institute of Materials Processing.
Material similar to that described here is no doubt readily available, and simply requires some oneÕs careful examination to find good crystals. One hand-sized sample can yield many micromounts. Similar material from Lime Crest was subsequently given to the author by Scott Stepanski. These samples contained graphite crystals in marble with morphologies very similar to those that are the focus in this paper, but associated with a somewhat different assemblage of minerals, which appears (but has not been confirmed) to include serpentine, zircon, muscovite, apatite and diopside, in addition to those already mentioned. The geology and mineralogy of Lime Crest are briefly described by Widmer (1962), Metsger (1977) and Tracy (1991).
Graphite Morphology
The crystal structure of graphite (Freise 1962) is that of a staggered stacking of sheets of carbon atoms (fig. 1). Within the sheets, the carbon atoms are very strongly bonded together in a honeycomb arrangement, with a C-C bond length of only 1.42 angstroms (1 angstrom = 10^{-8} cm), which is even closer than the C-C bond-length in diamond. In contrast, the carbon sheets are separated from each other by approximately 3.40 and are only weakly bonded to each other. This results in graphite's flexibility and softness. Both hexagonal and rhombohedral types (called polytypes) of graphite are known, depending on how the sheets are staggered in their stacking. The most common and most stable graphite polytype is the hexagonal, or 2H, polytype, which has its layers staggered in an ...ABABAB... sequence (as in fig. 1). The rhombohedral, or 3R, polytype has its layers staggered in an ...ABCABC... sequence (Laves and Baskin 1956). The rhombohedral polytype is only known as a mixture in predominantly hexagonal crystals, and can be induced to form by grinding hexagonal graphite.
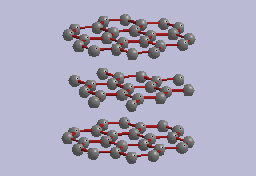 Fig. 1. Idealized crystal structure of hexagonal graphite showing sheets
of carbon atoms bonded in a honeycomb pattern. The c-axis of the graphite
is perpendicular to the sheets.
Fig. 1. Idealized crystal structure of hexagonal graphite showing sheets
of carbon atoms bonded in a honeycomb pattern. The c-axis of the graphite
is perpendicular to the sheets.
As a result of the crystal structure, the symmetry and the nature of the bonding, the {0001}
basal pinacoid faces (c-faces) of graphite crystals tend to be the lowest energy surfaces, and
growth of the crystals is typically fastest at the sheet edges. These factors lead to tabular hexagonal
plates as being the typical morphology of graphite crystals (Jaszczak 1995). By far the most
common crystal form for graphite at Lime Crest and elsewhere is the basal pinacoid (figs. 2 to 7).
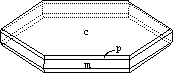 Fig. 2. Idealized graphite crystal showing the basal pinacoid c{0001},
the first-order prism m{10-10}, and the first-order dipyramid p{10-11}.
Fig. 2. Idealized graphite crystal showing the basal pinacoid c{0001},
the first-order prism m{10-10}, and the first-order dipyramid p{10-11}.
The c-faces of the graphite crystals from most localities are typically highly striated (fig. 5)
due to the crystals being mechanically bent and deformed by the various stresses and forces on and
in the host-rock. The bending of the crystals induces so-called "mechanical
twinning" that is
evidenced by the striations on c-faces (see Palache 1941). Many small graphite crystals from
Lime Crest, however, can be found with no striations on the c-faces, indicating their rare escape
from the many factors that could have deformed them. A few crystals have been observed that
show growth twins that are evident as small triangular crystals protruding from the c-faces, as
shown in fig. 3 (Jaszczak 1991).

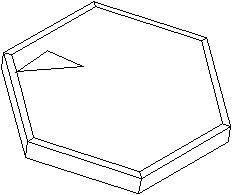
Fig. 3. (a) Graphite crystal (0.7mm) showing a smaller growth twin (brightly reflecting), twinned according to graphite's most common twin law: by reflection and contact on a {11-21} plane. (b) Idealized graphite crystal illustrating the same twin law.
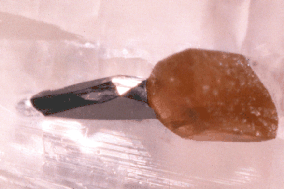
Fig. 4. A well-formed, lustrous graphite crystal (0.6 mm) showing
part of one of the typical basal pinacoid faces as well as
several modifying faces. The crystal is partially embedded in
calcite and associated with an orange-brown phlogopite crystal.
JAJ specimen #1556h and photo.

Fig. 5. An unusually thick graphite crystal with uncommon faces, on a basal pinacoid face of a more typical, striated, tabular graphite crystal (1.3 mm). JAJ specimen #1556i and photo.
A few graphite crystals from Lime Crest have been found that
display spiral growth steps on the c-faces.
The spirals can usually
only be observed by orienting the crystals to reflect the light "just right"
to make the steps visible, and are difficult to photograph (fig. 6). Such growth
spirals are thought to emerge from defects in the crystal structure
called screw dislocations, which play an important role in the growth of
the crystals (Weiner and Hager 1987). A crystal exhibiting a different of
spiral is shown in fig. 7. Rather than just having spiral steps on teh c-faces,
this entire crystal seems to be coiled as a macroscopic spiral, similar
to a few turns of a spiral staircase. The overall appearance suggests
that the growth of such "macrospirals" also might be related to
growth twinning (compare fig. 3).

Fig. 6. Graphite crystal (0.7mm) showing spiral steps on the c-face. Author's specimen #1626 and photo.

Fig. 7. Graphite crystal (0.7mm) showing a "macrospiral". Author's specimen #1626 and photo.
Occasionally graphite crystals will show well-formed prism faces. These are often striated as well, not because of mechanical twinning, but because of the graphite's layered crystal structure. Only rarely do other forms show themselves in well-formed, lustrous crystal faces (see figs. 4 and 5).

Fig. 8. A 1.1-mm crystal of molybdenite in partially etched calcite
with minor pyrite. JAJ specimen #1556e and photo.
Molybdenite, which can be easily confused with graphite, also occurs
in the marble at Lime Crest as well-formed, lustrous, platy crystals (fig. 8),
but is much less common. However, graphite and molybdenite crystals submerged
in a shallow bath of water and viewed with a microscope can readily be
distinguished from one another by the difference in their color. Compared to
graphite, the molybdenite shows a distinctly bluish hue. A few graphite
crystals have been found having molybdenite overgrown on graphiteÕs c-faces.
Although both the graphite and the molybdenite have their c-axes parallel in
these samples, an epitaxic relationship has not yet been confirmed in these
samples.
An interesting set of crystals that occur at Lime Crest shows secondary graphite overgrowths on earlier-formed, larger graphite crystals (figs. 9 to 11). Similar graphite overgrowths from Precambrian Grenville Series marble at Eagle Lake, Essex County, New York have been described by Weis (1980). Such overgrowths have also been noted by the author from the Sterling Mine, Ogdensburg, New Jersey, the Treadway Quarry, Port Henry, New York, the Limberg quarry, Pargas, Finland, a roadcut near Dresden, New York, and a roadcut south of Gooderham, Ontario, Canada. It is interesting to note that graphite occurs at all of these localities also as spherical aggregates in sizes ranging from microns in diameter at Pargas, up to a centimeter in diameter at the Sterling Mine. This correlation suggests a possible relationship between the growth mechanisms, growth conditions, and growth sequence of the graphite spheres and the graphite overgrowths.
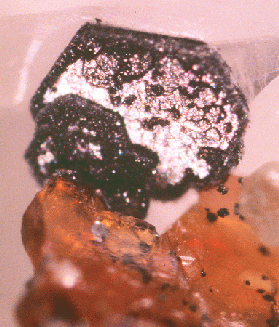
Fig. 9. A well-formed hexagonal graphite crystal (1.2 mm) with a thick secondary overgrowth of graphite. The crystal is on partially etched calcite and is associated with ora nge phlogopite that is included with tiny graphite spheres, and an unidentified pale green mineral at the mid-lower right. JAJ specimen #1556b and photo.
A whole spectrum of varying degrees of graphite overgrowth on earlier-formed graphite can be identified in material from Lime Crest. At one end of the spectrum there are the lustrous crystals with no overgrowth whatsoever. On some crystals, overgrowth is primarily localized on the crystal edges leaving areas of lustrous, earlier-formed graphite still visible (fig. 9). This suggests that either the secondary graphite growth was nucleated by the edges of the earlier- generation graphite, or perhaps that the secondary growth took place rapidly compared to the diffusion of carbon to the crystal surface. On other graphite crystals the whole surface can be overgrown by the second-generation graphite. At the opposite end of the spectrum are particularly thick overgrowths that seem to take on a spherical morphology (fig. 11), similar to crystals also observed by Weis. Whereas in the partially coated crystals the secondary graphite shows some degree of order in their alignment with each other and with the substrate crystal, the thickly coated crystals appear to have more randomly oriented overgrowths. The preservation of the delicate secondary crystallites and the undistorted morphology of the first-generation graphite crystals implies that the metamorphic processes that commonly deform rock and distort or destroy the enclosed graphite crystals must have been greatly diminished by the time the graphite crystals formed.
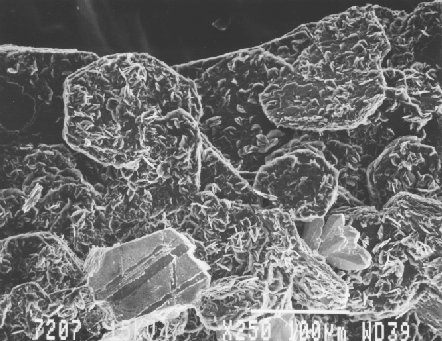
Fig. 10. SEM photo of hexagonal graphite crystals (to approximately 0.2 mm) showing asecondary overgrowth of graphite crystals as minute plates. Associated are a spray of arsenopyrite crystals at the lower right and a plate of pyrrhotite at the lower left. SEM photo by Ruth I. Kramer. JAJ specimen #1556.
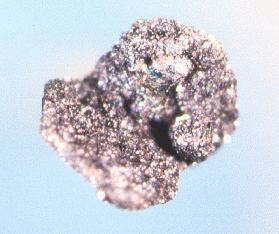
Fig. 11. A 1-mm graphite crystal (inclined) thickly overgrown by a second generation of graphite to such an extent that spherical clusters appear. JAJ specimen #1556g and photo.
Some of the most unusual graphites observed from Lime
Crest are pseudocubic aggregates of crystals that reach up to 0.5 mm
across and have a rather velvety luster (figs. 12 to 14). In
shape they are reminiscent of small balls of putty that have been
dropped several times on a flat surface, giving both rounded and
flattened areas. It appears, however, that the flattened areas form
90-degree angles with each other, as would cube faces.
The aggregates are composed of flat crystal flakes (figs. 13 and 14), which
show that the dominant growth mechanism is still fastest at the
edges of the graphite sheets. The cause of the overall pseudocubic
morphology could be pseudomorphism after some other mineral, although this
has not been verified.
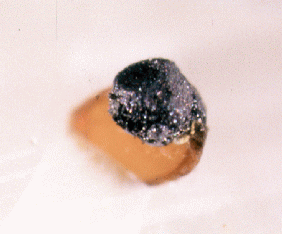
Fig. 12. An aggregate of graphite crystals (0.4 mm)
showing
a pseudo-cubic habit, with phlogopite on calcite.
JAJ specimen #1556d and photo.
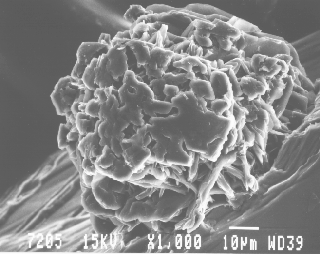
Fig. 13. SEM photo of a graphite pseudo-cube (nearly 0.1 mm) on phlogopite, partially etched from calcite. Individual graphite crystals in the aggregate seem to be partially aligned. SEM photo by Ruth I. Kramer. JAJ specimen #1556.

Fig. 14. SEM photo of a somewhat larger graphite pseudo-cube than shown in Fig. 13, viewed such that two of the cube sides are at the top left and at the right. In the area of the cube corner at the lower left, the individual graphite flakes appear to be randomly oriented. SEM photo by Ruth I. Kramer. JAJ specimen #1556.
Finally, distinctly spherical aggregates of graphite occur
up to 0.5 mm in diameter and are commonly clustered around phlogopite
crystals (fig. 15). It will be an interesting future study to investigate
if these spheres are related to the pseudocubic graphite aggregates or
possibly to spherical graphite that is known to occur at a number of
other localities, such as Sterling Hill and Franklin, New Jersey,
Chilson Hill, New York, Gooderham, Ontario, and others (Lemanski,
1991; Dunn 1995; Jaszczak 1995 and unpublished).

Fig. 15. Cluster of graphite spheres (up to 0.4 mm)
on orange-brown
phlogopite, partially etched from calcite.
JAJ specimen #1556c and photo.
Acknowledgments
I am grateful to John Rakovan and Scott Stepanski for collecting and providing bulk samples for this study. The Limestone Products Corporation of America is also gratefully acknowledged for allowing the collection of mineral samples. I also thank Pete Dunn, who supplied some useful references, and Michael A. Hawkins, who supplied material from the Treadway quarry for study. I thank Ruth I. Kramer and the Institute of Materials Processing at Michigan Technological University for SEM time and photographs.References
Chamberlain, S. C., King, V. T., Cooke, D., Robinson, G. W. and Holt, W. (1996) Minerals of the Gouverneur Talc No. 4 Quarry, Town of Diana, Lewis County, New York. Rocks & Minerals (in press).Dunn, P. J. (1995) Franklin and Sterling Hill, New Jersey: The World's Most Magnificent Mineral Deposits. Part 4. Franklin-Ogdensburg Mineralogical Society, Franklin, NJ. pp. 521-522.
England, B. M. (1991) The State of the Science: Scanning Electron Microscopy. Mineralogical Record 22, 123-132.
Freise, E. J. (1962) Structure of graphite. Nature (London) 193, 671-672.
Gerdes, M. L. and Valley, J. W. (1994) Fluid flow and mass transport at the Valentine wollastonite deposit, Adirondack Mountains, New York State. Journal of Metamorphic Geology 12, 589-608.
Jaszczak, J. A. (1991) Graphite from Crestmore, California. Mineralogical Record 22, 427-432.
Jaszczak, J. A. (1994) Famous graphite crystals from Sterling Hill, New Jersey. The Picking Table 35 (2), 6-11.
Jaszczak, J. A. (1995) Graphite: Flat, Fibrous and Spherical. In, Mesomolecules: From Molecules to Materials, edited by G. D. Mendenhall, J. Liebman and A. Greenberg (Chapman & Hall, New York). pp. 161-180.
Jaszczak, J. A. (1996) Morphology of graphite from Pargas (Parainen), Finland. Program of the 23rd Rochester Mineralogical Symposium, Rochester, New York, April 18-21, 1996; Rocks & Minerals (in press).
Lemanski, C. S., Jr. (1991) Graphite in ore: An unusual occurrence at the Sterling Mine. Picking Table, 32 (1), 11-13.
Laves, F. and Baskin, Y. (1956) On the formation of the rhombohedral graphite modification. Zeitschrift fur Kristallographie 107, 337-35.
Metsger, R. W. (1977) Notes on the Precambrian Metalimestones of Northern New Jersey. In, Stratigraphy and Applied geology of the Lower Paleozoic Carbonates in Northwestern New Jersey: Guidebook for the 42nd Annual Field Conference of Pennsylvania Geologists, October 6-8, 1977. (Field Conferences of Pennsylvania Geologists, c/o Bureau of Topographic & Geologic Survey, Department of Environmental Resources, Harrisburg, PA) pp. 48-54.
Palache, C. (1941) Contributions to the mineralogy of Sterling Hill, New Jersey: Morphology of graphite, arsenopyrite, pyrite, and arsenic. American Mineralogist 26, 709-717.
Tracy, R. J. (1991) Ba-rich micas from the Franklin Marble, Lime Crest and Sterling Hill, New Jersey. American Mineralogist 76, 1683-1693.
Weiner, K.-L. and Hager, H. (1987) [Growth spirals on graphite crystals.] Lapis 12 (1), 31-33. (In German).
Weis, P. L. (1980) Graphite skeleton crystals- A newly recognized morphology of crystalline carbon in metasedimentary rocks. Geology 8, 296-297.
Widmer, K. (1962) The Limecrest Quarry. In, Northern Field Excursion Guidebook- International. Mineralogical Association, 3rd General Congress, (Mineralogical Society of America, Washington, D.C.) pp. 22-23.
 Back to MTU Physics Page
Back to MTU Physics Page Back to Jaszczak's home page
Back to Jaszczak's home page
 Back to Jaszczak's graphite page
Back to Jaszczak's graphite page