Published in The Picking Table 35(2), 6-11 (1994).
FAMOUS GRAPHITE CRYSTALS
FROM STERLING HILL, NEW JERSEY
John A. Jaszczak
Department of Physics and the Seaman Mineral Museum
Michigan Technological University
1400 Townsend Dr.
Houghton, Michigan 49931-1295
In 1941, Charles Palache published an important
paper on the
morphology of graphite crystals from Sterling Hill. One of his graphite
crystal drawings, which has been reproduced in many publications, is
drawn down the c-axis and gives the impression of being tabular.
However, the given Miller indices indicate that the crystal was actually
barrel-shaped, a rare morphology for graphite.
INTRODUCTION
In 1941, Charles Palache of Harvard University
published a paper [hereafter referred to as CP41] titled,
Contributions to the Mineralogy of Sterling Hill, New
Jersey: Morphology of Graphite, Arsenopyrite, Pyrite, and
Arsenic" in The American Mineralogist. The morphology
of graphite was the focus of the paper, although the other
listed minerals are illustrated and discussed to a minor
degree. Historically, the symmetry of graphite had been the
subject of some controversy. By goniometric measurements
of the crystals from Sterling Hill, Palache correctly
concluded that graphite is hexagonal with full symmetry.
CP41 has since been cited in at least 29 articles and
books in the fields of mineralogy, physics, metallurgy and
materials science, and includes publications in German,
Russian and English (see Appendix). The paper contains
five crystal drawings of Sterling Hill graphite, one of
Ticonderoga, New York graphite, four of Sterling Hill
arsenopyrite, and one of Sterling Hill native arsenic. Two of
the graphite crystal drawings have since been reproduced in
other publications. Those of the Ticonderoga crystal (CP41
fig. 5) and one of the Sterling Hill crystals (CP41 fig. 3;
see fig. 1 of this paper) appeared in the seventh edition of
Dana's System of Mineralogy, which Palache co-authored
(Palache, et al. 1944). Reproduction of the figures in Dana's
System has probably contributed greatly to Palache's
drawings being widely regarded as the "textbook" examples
of graphite crystals. Along with a sketch of a synthetic,
scroll-type, whisker crystal of graphite, fig. 3 of CP41 also
appeared on the cover of the book Preparation and Properties
of Solid State Materials, vol. 4. (Wilcox, 1979). It was
again reproduced, along with fig. 5 of the Ticonderoga
crystal, in the book's first chapter on "Graphite
Crystallization", written by I. Minkoff. In the book The
Physical Metallurgy of Cast Iron by Minkoff (1983), fig. 5
of the Ticonderoga, NY crystal was included once more, but
fig. 3 of the Sterling Hill graphite was not.

Fig. 1. Crystal drawing of graphite from Sterling Hill, NJ,
which appeared originally as fig. 3 in Palache's 1941 paper
in American Mineralogist. The crystal shows the basal
pinacoid c{0001}, the first-order dipyramid p{10-11} and the
second-order dipyramid phi{11-22}. (Drawing recreated using
the computer program SHAPE.)
GRAPHITE CRYSTALS
According to Palache, the source of the studied crystals
was Mr. Lawson H. Bauer of Franklin, New Jersey, who
found and carefully isolated the graphite crystals from a
coarsely crystalline marble impregnated with graphite,
arsenic, realgar, pyrite, arsenopyrite, diopside and either
stibnite or a lead sulphantimonide [probably zinkenite (Kolic
and Sanford, 1993)]; the specimens were collected May,
1937 from the 900-foot level of the mine at Sterling Hill.
Dilute hydrochloric acid was used to separate these minerals
in abundance from the marble matrix. In 1937 and in
following years Bauer made contributions of isolated
graphite crystals to the Harvard Mineralogical Laboratory.
Mr. Bauer is said to have been a generous, careful and patient
enthusiast of Franklin minerals who at one time became
very fond of graphite (J. L. Baum, personal communication,
1993; Frondel, 1955).
Overall, the graphite crystals described by Palache in
CP41 are considered to have been of superior quality. For
example, experts in the laboratory synthesis of synthetic
graphite crystals (Austerman, et al., 1967) stated:
The natural crystals described by Palache
are the highest quality crystals reported
in the literature as far as the authors are
aware, and his description serves as a
point of reference for the graphite
crystals grown in the laboratory.
Palache described the Sterling Hill graphite crystals as
being up to 1 or 2 mm in diameter, while the best crystals
did not exceed 0.5 mm. Although hundreds of graphite
crystals were examined by him, only ten were completely
measured by optical goniometry. From these 10 crystals,
Palache listed 15 sets of measured angles corresponding to
16 different crystal forms (see table 1). Palache noted that
most of the crystals had been distorted as a result of tectonic
deformation, which caused mechanical twinning of both the
graphite and the surrounding calcite. Few graphite crystals
remained undistortied or showed only a single twin lamella.
TABLE 1: Graphite crystal forms measured by Palache
(1941) from a set of 10 crystals from Sterling Hill, NJ. The
basal pinacoid {0001} was used for alignment of the crystals
on the goniometer and was not included in table 1 of CP41.
Presumably at least one {0001} faced existed on each of the
10 crystals.
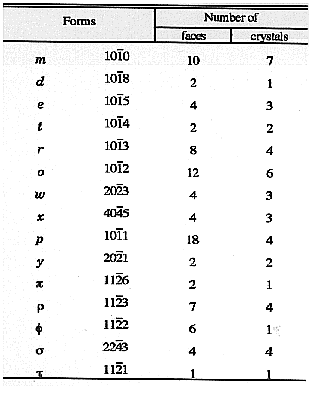
The most common form observed by Palache on
graphite crystals from Sterling Hill was the basal pinacoid
{0001}, which he described as dominant and highly lustrous.
The same form is the most common in graphite form almost
all other localities woldwide. After that, the first order
dipyramids {10-11} and {10-12}, which determined the
hexagonal outline of the crystals, were about equally as
common. The {10-13} dipyramid was slightly less common,
and other dipyramids less common still. Palache noted that
only rarely did any one of the dipyramids show all 6 faces on
(the top half of) a crystal, and the zone of these faces was
often completely striated. The first order prism {10-10} was
rarely more than a line face, always rough when present, but
often missing altogether. Second order dipyramids, such as
{11-22}, occurred as small faces such as truncations of first
order dipyramid faces. It is noteworthy that none of the
forms listed by Palache corresponds to a general form {hkil}
(were h, k, and l are non-zero and have no special
relationship to one another), as general forms of graphite not
been well-documented in subsequent literature either.
Palache did note, however, the existence of crystal faces
which were not in the dominant zones and could not be
simply indexed.
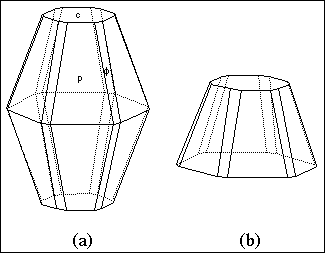
Fig. 2. (a) Same crystal as in fig. 1 but rotated to a
different viewing orientation using SHAPE. (b) Cleaved
version of (a) which also appears like fig. 1 when viewed
down the c axis, but is more apt to fit Palache's description
of the original crystal.
Graphite usually forms tabular crystals, when it forms
good crystals at all. Of the crystal drawings of graphite in
Palache's paper, two showed the crystals from an inclined
view and clearly are tabular. Palache indicates that at least
figures 1, 2 and 3 represent measured crystals with the
relative prominence of faces in the drawings approximately
corresponding to that of the actual crystals. It is therefore
easy to assume that the crystal depicted in fig. 3 of CP41
(see fig. 1 above) is also tabular. However, as determined
from the Miller indices given in the paper, along with the
angles of inclination of the faces from the c-axis, this cannot
be the case. Using the axial dimensions given by Palache
and the Miller indices corresponding to the crystal illustrated
in fig. 3 of his paper, the Macintosh version of the crystal-
drawing program SHAPE (Dowty and Richards, 1993) was
used to redraw the crystal. The sizes of the faces were
adjusted to match Palache's fig. 3 when viewed directly down
the c-axis (fig. 1 above). SHAPE was then simply used to
rotate the crystal to be viewed away from the c-axis,
whereupon its barrel-shaped morphology immediately
became apparent (see fig. 2a). Such a morphology for
graphite is very uncommon [compare, however, Kvasnitsa et
al. (1988)]. As Palache mentioned that most of the crystals
did not have well-developed faces on both the top and bottom
halves, the crystal corresponding to Palache's fig. 3 might
be only a fraction of that depicted in fig. 2a, as shown in fig.
2b. Unfortunately, attempts to locate this or any of the
other studied graphite crystals at the Harvard Mineralogical
Museum have failed. A batch of insoluble residues from
Bauer has been preserved in a collection recently donated by
John L. Baum (J. L. Baum, personal communication, 1992)
to the U.S. National Museum of Natural History, but is
currently unavailable for study (P. J. Dunn, personal
communication, 1993).
 .
.
Post-Publication Note:1-mm Graphite crystals from acid
residues in the L.H. Bauer collection in
the Smithsonian do show the barrel-shaped habit.
TWINNING
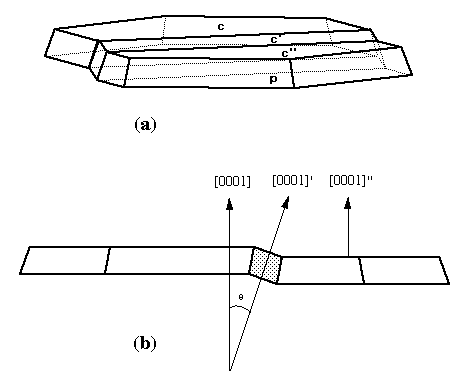
(c) 
Fig. 3. (a) Graphite crystal from Sterling Hill (fig. 6 in
CP41 recreated using SHAPE) with forms c{0001} and
p{10-11}, showing a single twin lamella on {11-21}. The
bottom of the crystal is a cleavage plane. (b) Side view of
(a) showing that the twin lamella (shaded) is inclined by
theta=20deg 36' from the adjacent, untwinned parts of the same
crystal. (c) Graphite crystal from Bauer residues donated to
the Smithsonian (National Museum of Natural History)
Palache sought crystals that were free from striations for
goniometric measurement. Striations are commonly
observed on the basal faces of graphite crystals and have long
been recognized as due to mechanical twinning (Sjšgren,
1884). These striations always are oriented diagonally to the
hexagonal outline, i.e., along <1-100> directions, and can be
induced by the application of only the slightest stress; thus,
untwinned, unstriated crystals are rare. By observing an
approximate 20deg angle between the basal pinacoids of a twin
lamella and the adjacent, untwinned parts of the same crystal,
Palache determined that the twin law is by reflection and
composition on {11-21} planes (see fig. 3). Palache was
apparently the first to identify the twin law, and such is the
reason that a large number of subsequent authors have cited
CP41. Palache also noted a twin lamella at an angle 16deg 43',
corresponding most nearly to a {44-83} twin plane. Growth
twins on this twin law as well as other twin laws are now
also known (Laves and Baskin, 1956; Freise and Kelly,
1961; Shafranovskii, 1981, 1982, 1983; Jaszczak, 1991,
1992) from several localities.
CONCLUSION
Charles Palache's 1941 paper was not only an important
contribution to the mineralogy of Sterling Hill, New Jersey,
but was also a unique and important contribution to the
descriptive mineralogy of graphite. The natural crystals
described and drawn by Palache are a recognized standard for
well-formed graphite crystals and are still considered to be
some of highest quality crystals reported in the literature.
Information regarding the existence or availability of
Palache's graphite crystals or additional material such as that
etched by Bauer would be greatly appreciated.
ACKNOWLEDGMENTS
I am most grateful to John L. Baum of the Franklin
Mineral Museum for providing information and
encouragement to write this article. I am indebted to Dr.
Carl Francis for assistance in trying to locate the graphite
crystals in the Harvard Mineralogical Museum collection. I
am grateful to Sharon Cisneros for bringing the reference by
Edwards (1976) to my attention.
REFERENCES
Austerman, S. B., Myron, S. M., and Wagner, J. W. (1967)
Growth and characterization of graphite single crystals.
Carbon 5, 549-557.
Dowty, E., and Richards, R. P. (1993) SHAPE, a computer
program for drawing crystals. Macintosh Version 4.0.
Freise, E. J., and Kelly, A. (1961) Twinning in graphite.
Proceedings of the Royal Society (London) A 264, 269-
276.
Frondel, C. (1955) Memorial of Lawson H. Bauer. American
Mineralogist 40, 283-285.
Jaszczak, J. A. (1991) Graphite from Crestmore, California.
Mineralogical Record 22, 427-431.
Jaszczak, J. A. (1992) Growth twinning in graphite from
Crestmore and Jensen quarries, Riverside County, California.
Rocks and Minerals 67, 114-115. (Abstract.)
Kolic, J. and Sanford, S. (1993) Recent mineral finds from
the Sterling Mine, Ogdensburg, New Jersey. The Picking
Table 34 (2), 12-21.
Kvasnitsa, V. N., Krochuk, V. M., Melnikov, V. S., and
Yatsenko, V. G. (1988) [Crystal morphology of graphite
from magmatic rocks from the Ukrainian Shield.]
Mineralogicheskii Zhurnal 10(5), 68-76. (In Russian with
English summary.)
Laves, F., and Baskin, Y. (1956) On the formation of the
rhombohedral graphite modification. Zeitschrift fuŸr
Kristallographie 107, S.337-356.
Minkoff, I. (1979) Graphite crystallization. In, Preparation
and Properties of Solid State Materials, vol. 4.
Morphological Stability, Convection, Graphite, and
Integrated Optics. Wilcox, W. R., editor. (Marcel Dekker,
New York) 1-48.
Minkoff, I. (1983) The Physical Metallurgy of Cast Iron.
(John Wiley and Sons, New York) p. 9.
Palache, C. (1941) Contributions to the mineralogy of
Sterling Hill, New Jersey: Morphology of graphite,
arsenopyrite, pyrite, and arsenic. American Mineralogist 26,
709-717.
Palache, C., Berman, H., and Frondel, C. (1944) The
System of Mineralogy of James Dwight Dana and Edward
Salisbury Dana, Yale University 1837-1892. Seventh
edition, vol. 1. (John Wiley & Sons, New York) 152.
Shafranovskii, G. I. (1981) [New graphite twins.] Zapiski
Vsesoyuznogo Mineralogicheskogo Obschestva 110, 716-
720. (In Russian.)
Shafranovskii, G. I. (1982) [Graphite twins and triads.]
Mineralogicheskii Zhurnal 4(1), 74-81. (In Russian with
English summary.)
Shafranovskii, G. I. (1983) [Classical and non-classical
graphite twins.] Zapiski Vsesoyuznogo Mineralogicheskogo
Obschestva 112, 577-581. (In Russian.)
Sjošgren, H. (1884) Om grafitens kristallform och fysiska
egenskaper. …fversigt af Svenska Vetenskapsakademien
Forhandlingar 44, 29-53.
Wilcox, W. R. editor (1979) Preparation and Properties of
Solid State Materials, vol. 4. Morphological Stability,
Convection, Graphite, and Integrated Optics. (Marcel
Dekker, New York).
APPENDIX
Chronologic list of publications in which Palache's 1941
paper (American Mineralogist 26, 709-717) has een cited
[not including this paper]. As this list is almost certainly
incomplete, the author invites notification of additional
publications that should be included.
1. Valter, A. A., Eremenko, G. K., Kvasnitsa, V. N., and
Polkanov, Yu. A. (1992) Udarno-
Metamorphogennure Mineralur Ugleroda [Shock-
Metamorphic Minerals of Carbon.] (Naukova Dumka,
Kiev) 172 pp. (In Russian.)
2. Jaszczak, J. A. (1992) Growth twinning in graphite
from Crestmore and Jensen quarries, Riverside County,
California. Rocks and Minerals 67, 114-115.
(Abstract.)
3. Pengra, D. B., and Dash, J. G. (1992) Edge melting in
low-coverage adsorbed films. Journal of Physics-
Condensed Matter 4, 7317-7332.
4. Jaszczak, J. A. (1991) Graphite from Crestmore,
California. Mineralogical Record 22, 427-432.
5. Kvasnitsa, V. N., Krochuk, V. M., Melnikov, V. S.,
and Yatsenko, V. G. (1988) [Crystal morphology of
graphite from magmatic rocks from the Ukrainian
Shield.] Mineralogicheskii Zhurnal 10(5), 68-76. (In
Russian with English summary.)
6. Minkoff, I. (1983) The Physical Metallurgy of Cast
Iron. (John Wiley and Sons, New York). Includes
figure 5, but not figure 3, from Palache (1941) on page
9.
7. Shafranovskii, G. I. (1982) [Crystallomorphology of
graphite from the Ilmen Mountains.] In,
Mineralogicheskie Issledovaniya Endogennurkh
Mestorozhdenii Urala. (Academiya Nauk SSSR-
Uralskii Nauchnuri Tsentr) 44-53. (In Russian.)
8. Shafranovskii, G. I. (1982) [Graphite twins and triads.]
Mineralogicheskii Zhurnal 4(1), 74-81. (In Russian
with English summary.)
9. Shafranovskii, G. I. (1981) [New graphite twins.]
Zapiski Vsesoyuznogo Mineralogicheskogo
Obschestva 110, 716-720. (In Russian.)
10. Minkoff, I. (1979) Graphite crystallization. In
Preparation and Properties of Solid State Materials,
vol. 4. Morphological Stability, Convection, Graphite
and Integrated Optics. Wilcox, W. R., editor. (Marcel
Dekker, New York) 1-48. Includes figures 3 (also on
the book cover) and 5 from Palache, 1941.
11. Munitz, A., and Minkoff, I. (1978) 45th International
Foundry Congress Paper (Hungary). See Peiyue, Z.,
Rozeng, S., and Yanxiang, L. (1985) Effect of twin/tilt
on the growth of graphite. In, The Physical Metallurgy
of Cast Iron, Fredriksson, H. and Hillert, M., editors.
MRS Symposium Proceedings, vol. 34. (North
Holland, New York) 3-11.
12. Edwards, F. Z. (1976) The post Palache minerals. The
Picking Table 17 (2), 6-10. ÒIt is quite evident that
Palache's interest was primarily with the graphite
crystals imbedded in the calcite. It is also probable that
the specimens submitted by Dr. Bauer were all
consumed by acid. At any rate, very few pieces of this
occurrence may be found in collections today.Ó p. 7.
"It should also be mentioned that equally scarce and
much unappreciated are the graphite crystals which so
intrigued Dr. Palache." p. 8.
13. Akhmatov, Y. S., Bunin, K. P., and Taran, Y. N.
(1975) [Mechanism of formation of graphite
spherocrystals in Fe-Ce melt.] Dopovidi Akademii
Nauk Ukrainskoi RSR Seriya A- Fiziko-Matematichni
Ta Technichninauki (5), 453-455. (In Russian.)
14. Nagornyi, V. G., Nabatnikov, A. P., Frolov, V. I.,
Deev, A. N., and Sosedov, V. P. (1975) [Occurrence of
a new crystalline form of carbon.] Zhurnal Fizicheskoi
Khimii 49(4) 840-845. (In Russian.)
15. Akhmatov, Y. S., Taran, Y. N., Stepanchuk, A. N.,
Lisnyak, A. G., and Zaspenko, N. Y. (1974) Structure
and mechanism of growth of graphite crystals with
different genetic origins. Russian Metallurgy- USSR
(4), 58-60.
16. Skinner, J., and Gane, N. (1973) The deformation and
twinning of graphite crystals under a point load.
Philosophical Magazine 28, 827-837.
17. Frondel, C. (1972) The Minerals of Franklin and
Sterling Hill: A Check List. (Wiley-Interscience, New
York) p. 58. ÒTiny but unusually perfect graphite
crystals have been found in acid-insoluble residues of
the marble-- see Palache (1941).Ó
18. Double, D. D., and Hellawell, A. (1969) The structure
of flake graphite in Ni-C eutectic alloy. Acta
Metallurgica 17, 1071-1083.
19. Gmelins Handbuch der Anorganischen Chemie (1968)
System-nummer 14. C- Kohlenstoff. Teil B, Lieferung
2. Das Element: Graphit. (Verlag Chemie, GMBH,
Weinheim/Gergstr.) 409, 412. (In German.)
20. Austerman, S. B., Myron, S. M., and Wagner, J. W.
(1967) Growth and characterization of graphite single
crystals. Carbon 5, 549-557.
21. Baker, C., Gillin, L. M., and Kelly, A. (1966)
Twinning in graphite. Second Conference on Industrial
Carbon and Graphite. (Society of Chemical Industry,
London) 132-138.
22. Amelinckx, S., Delavignette, P., and Heerschap, M.
(1965) Dislocations and stacking faults in graphite. In,
Chemistry and Physics of Carbon, Walker, P. L., Jr.,
editor. (Marcel Dekker, New York) 1-71.
23. Thomas, J. M. (1965) Microscopic studies of graphite
oxidation. In, Chemistry and Physics of Carbon,
Walker, P. L., Jr., editor. (Marcel Dekker, New York)
121-202.
24. Freise, E. J., and Kelly, A. (1961) Twinning in
graphite. Proceedings of the Royal Society (London) A
264, 269-276.
25. Kennedy, A. J. (1960) Dislocations and twinning in
graphite. Proceedings of the Physical Society (Great
Britain) 75, 607-611.
26. Academiia Nauk SSSR. Institut Geologii Rudnykh
Mestorozhdenii, Petrografii, Mineralogii i Geokhimii.
(1960) Mineraly; Spravochnik, vol. 1. (Izd-vo
Academii Nauk SSSR, Moscow) 69-75. (In Russian.)
27. Foster, L. M., Long, G., and Stumpf, H. C. (1958)
Production of graphite single crystals by the thermal
decomposition of aluminum carbide. American
Mineralogist 43, 285-296.
28. Platt, J. R. (1957) Atomic arrangements and bonding
across a twinning plane in graphite. Zeitschrift fŸr
Kristallographie 109, S.226-230.
29. Palache, C., Berman, H., and Frondel, C. (1944) The
System of Mineralogy of James Dwight Dana and
Edward Salisbury Dana, Yale University 1837-1892.
Seventh edition, vol. 1. (John Wiley and Sons, New
York) 152. Includes figures 3 and 5 from Palache,
1941.
 Back to MTU Physics Page
Back to MTU Physics Page
 Back to Jaszczak's home page
Back to Jaszczak's home page
 Back to Jaszczak's graphite page
Back to Jaszczak's graphite page






 Back to MTU Physics Page
Back to MTU Physics Page