Exam #1: February
17, 2005
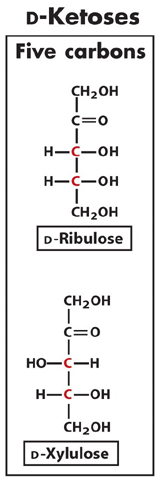
1. Using the Fischer projection, draw and name any
five-carbon D-ketose.
2. Draw the cyclization reaction of any D-aldohexose
and name it. How many asymmetric carbons (chiral centers) does this
structure have? How many stereoisomers are theoretically possible?
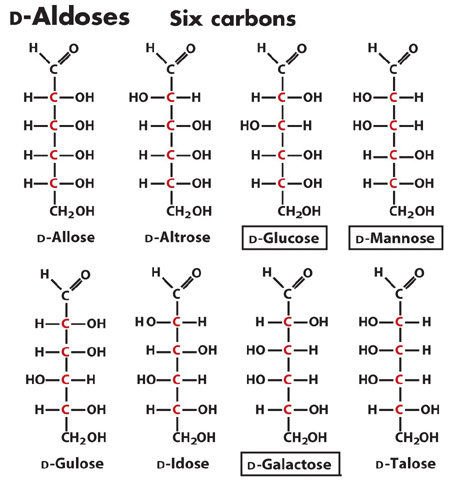
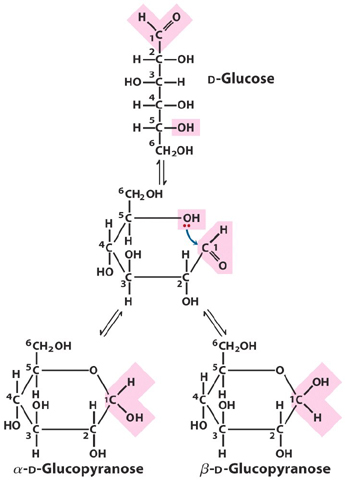
There are 5 asymmetric carbons. Therefore 2^5 stereoisomers (32).
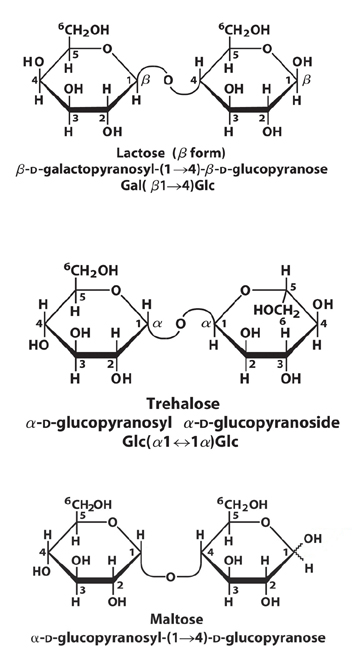
3. Draw and name a disaccharide.
4. Identify and describe the biological roles of
three (3) polysaccharides.
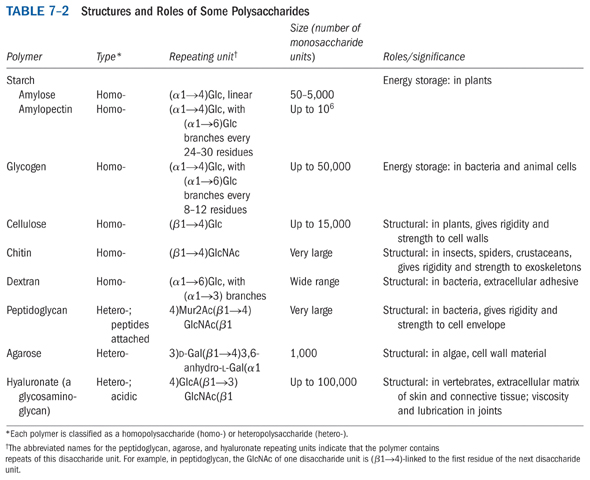
5. What is the difference between a proteoglycan and
a glycoprotein?
Both are made up of proteins and
saccharides. Difference is that in proteoglycans, carbohydrates make up
roughly 95% of the mass. whereas
in glycoproteins the bulk of the biomolecule is protein.
6. What types of linkages connect oligosaccharides to
proteins? Draw each using one monomeric unit from each biomolecule.
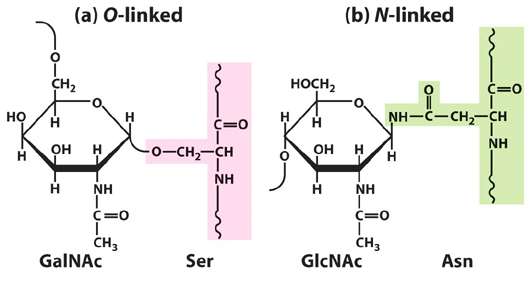
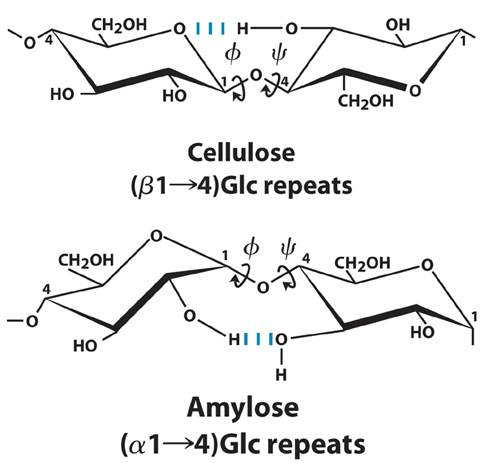
7.
Cellulose and amylose are both homopolymers of glucose, yet they adopt
different 3-dimensional structures.
a. Explain.
a1-->4 linkage permits formation of helical structures,
whereas the
b1-->4 linkage forms a linear structure
b. Using two
representative sugars, draw the two structures to illustrate the
differences (include hydrogen bonding, label the dihedral angles)
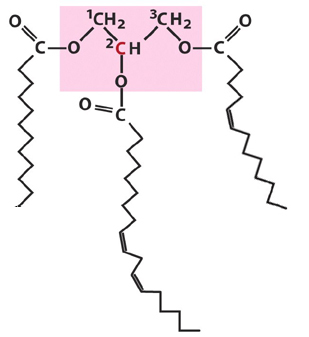
8. Draw a fat containing 12:0, 16:2 (D9,12) and
14:1(D5).
9. Why do fats make good storage fuels and
polysaccharides make good sources of quick energy?
Nonpolar so dont hydrate. Low
density, low MW for storage.
10. Why would storage lipids make bad structural
lipids?
nonpolar, whereas structural
lipids are amphipathic
11. How does heparin work to inhibit blood
coagulation?
Inhibits blood coagulation by
binding to the protein antithrombin, causing antithrombin to bind and
inhibit thrombin, a protease essential for clotting
12. Define amphipathic.
a macromolecule with polar and
nonpolar regions
13. Draw a glycerophospholipid and a sphingophospholipid, where
an amino acid is coordinated via phosphodiester linkage. Use a
different amino acid for each.
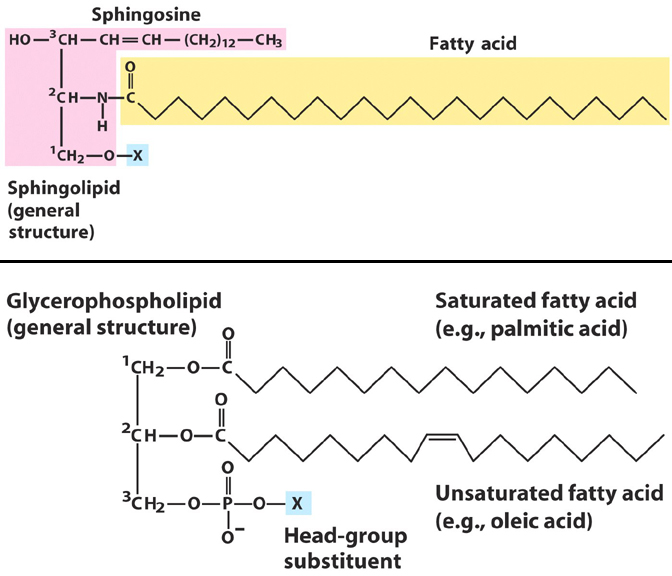
The 'X' group for each should be
any amino acid with a hydroxyl side
chain (Ser, Thr, Tyr)
14. Describe how the following affect the
solid/liquid transition temperature of a membrane:
a. Increase on percent of unsaturated fatty acids
decreases packing, order and temp
b. Decrease in acyl chain length for saturated fatty
acids
decreases facial interactions
(smaller S.A.), therefore a bit less order and decreases temp
c. Sterol content is increased
Orders and stabilizes fatty acid
chains, increases order and temp
15. What are membrane rafts and in what ways do they
vary compared to the surrounding sea (non-raft) portions of a membrane?
aggregation of long chain
sphingolipids, which then select for certain proteins, steroids, etc
that further make it a rigid cohesive structure
16. *Describe
a method of studying membrane dynamics and what it would tell you.
FRAP
17. *Describe
how you would purify a membrane protein?
Depends on desription.
18. What are the two components of an electrochemical
gradient?
1. electrical gradient
2. chemical gradient
19. Describe the following:
a. Passive transport
Proteins provide a path for
charged or polar compounds through the membrane. Usually a downhill
process.
b. Electrochemical gradient
sum of electrical and chemical
gradient across membrane
c. Antiport process
two substrates moving in opposite
directions across a membrane. Can be used to balance charge or couple a
thermodynamically unfavorable process to on that is favorable.
d. Secondary active transport
Encompasses antiport process.
Occurs when an endergonic (uphill) transport of one solute is coupled
to the exergonic (downhill) flow of a different solute that itself was
originally pumped uphill by primary active transport.
20. Calculate the free energy of transport of Ca2+
from inside an organelle and out into the cytoplasm, where:
a. = -85mV
b. R=8.315J/K mol, F = 96,480 J/V mol
c. T@30C
d. [Ca2+]in = 140mM, [Ca2+]out = 8mM
-7.2 kJ/mol +(-16.4 kJ/mol) = -23kJ/mol








