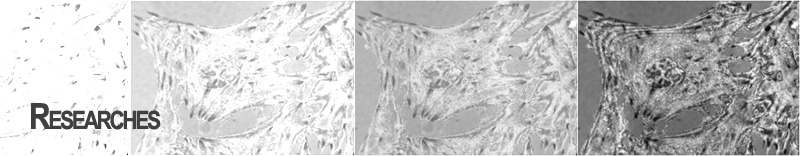
Protein tracking and detection

Since beta-catenin, which plays a critical role in intercellular adhesion and signal transduction between cells, has been known to be determined to form complexes with APC, the tumor suppressor gene, it is strongly believed to be related to tumorgenesis and regulation of gene activity. HCT-116 cells were used to transfected with pEGFP-beta-catenin construct, and the integrated dynamic live-cell imaging system was used to detect protein expression. As shown in this figure, dynamic TIRFM images can provide beta-catenin distributions even in a single cells and thus these images plays an important role on analyzing time series of beta-catenin interaction with cell-cell adhesion cadherin proteins, transcription factors, and other proteins such as axin. For example, iectopic beta-catenin protein was highly expressed in the cytoplasm and less expression was found in the nuclei. However, as time goes, the intensity of protein was diminished and moved to plasma membrane, indicating that transfected beta-catenin is degraded and bind to protein located in the plasma membrane, such as E-cadherin.
Even until now, there has not been clearly known the mechanism that the tumor suppressor functions of APC is assumed to rely upon its ability to bind beta-catenin and to facilitate beta-catenin degradation. The combination of EFM and TIRFM in addition to BFM in the integrated dynamic live-cell imaging system is believed to an effective tool to dynamically examine translocation of beta-catenin from cell membrane to cytoplasm, cytoplasm to cell membrane and/or nuclear membrane, and/or nuclear membrane to cytoplasm and/or cellular membrane as well as degradation of beta-catenin.
This work is sort of in the state of hiatus but Dr. Choi is eager to resume tracking proteins with a help of Dr. Beak in the University of Tennessee, Knoxville TN.