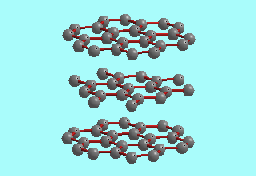 |
Graphite |
| Structure of 2H polytype |
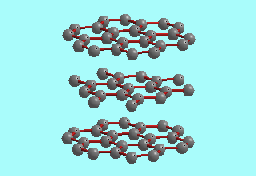 |
Graphite |
| Structure of 2H polytype |
The paradigm for the structure of graphite is that of a staggered stacking of flat layers of carbon atoms (see above). Individual layers, sometimes referred to as ``graphene" sheets (Dresselhaus et al., 1988)), are weakly bonded to each other, and are composed of strongly bonded carbon atoms at the vertices of a network of regular hexagons in a honeycomb pattern (Freise, 1962). Both hexagonal and rhombohedral polytypes of graphite are known depending on how the graphene sheets are staggered. The hexagonal or 2H polytype has the layers staggered in an ABAB... sequence, while the rhombohedral or 3R polytype has the layers stacked in an ABCABC... sequence.
Both the properties and the morphology of graphite reflect its highly anisotropic structure. Due to the strong bonding within the layers and weak bonding between layers, the growth of graphite takes place predominantly along the edges of the layers (perpendicular to the c-axis) and only very slowly normal to the layers (parallel to the c-axis). As a result of the growth rate anisotropy, the anisotropic surface energy, and the crystallographic symmetry, the expected morphology for graphite crystals is that of tabular hexagonal prisms (Schneer, 1977). However, well formed natural crystals such as shown below, are in fact quite rare (Jaszczak, 1991). Well formed laboratory-grown crystals of graphite are also uncommon. During the 1960's, graphite crystals from Ticonderoga, New York and Sterling Hill, New Jersey, became a standard of perfection for experiments and for comparison with laboratory-grown crystals.(Palache, 1941; Austerman, 1968; Jaszczak, 1994)
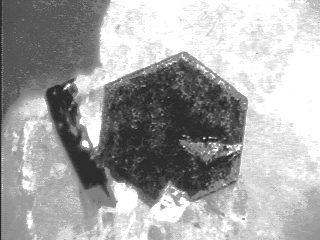 |
pyramid faces, twinned on {11-21}, in calcite. |
 |
Click here to compare other spirals. |
Like graphite, diamond
is also a mineral composed solely of the
element carbon. Unlike graphite, however, the strong bonding between carbon
atoms in diamond is not confined to layers, rather, the bonds in diamond
are pointed toward the vertices of a regular tetrahedron. This results
in a very dense structure for dimond, and a
relative to graphite, a much
more isotropic structure. Whereas graphite crystallizes in the hexagonal
crystal system, diamond crystallizes in the isometric (cubic) system. The
difference in the bonding, despite having only carbon atoms, results in
a profound difference in the properties of graphite and diamond:
Whereas
 |
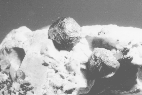
Udachnaya Mine, Republic of Sakha (Former Soviet Union). |
One of the most unusual morphologies of graphite is that of spheres
that have a radial texture in cross section.
Click here for more.
Compare:
Excellent microcrystals, crystals with secondary graphite overgrowths and even spherical aggregates of graphite occur in the Franklin Marble at the Lime Crest quarry near Sparta, New Jersey.
AMARI, S., HOPPE, P., ZINNER, E. and LEWIS, R.S. (1993) Nature (London) 365, 806.
AUSTERMAN, S.B. (1968) Growth of graphite crystals from solution. In, Chemistry and Physics of Carbon, vol. 4. P.L. Walker, Jr., editor. (Marcel Dekker, New York) p. 137-183.
DRESSELHAUS, M.S., DRESSELHAUS, G., SURIHARA, K., SPAIN, I.L., and GOLDBERG, H.A. (1988) Graphite Fibers and Filaments, (Springer, Berlin).
FREISE, E.J. (1962) Structure of graphite. Nature (London) 193, 671-672.
JASZCZAK, J.A. (1991) Graphite from Crestmore, California. Mineralogical Record 21, 427-432.
JASZCZAK, J.A. (1994) Famous graphite crystals from Sterling Hill, New Jersey. The Picking Table 35, (2), 6-11.
JASZCZAK, J.A. (1995) Graphite: Flat, Fibrous and Spherical. In, Mesomolecules: From Molecules to Materials. G.D. Mendenhall, A. Greenberg, and J.F. Liebman, editors. (Chapman & Hall, New York) pp. 161-180.
PALACHE, C. (1941) Contributions to the mineralogy of Sterling Hill, New Jersey: morphology of graphite, arsenopyrite, pyrite, and arsenic. Amercan Mineralogist 26, 709-717.
RUSSELL, A. (1988) Graphite: current shortfalls in flake supply. Industrial Minerals. (255), 23-43.
SCHNEER, C.J. (1977) editor. Crystal Form and Structure. (Dowden, Hutchison & Ross, Stroudsburg, PA) 368 pp. A good review of the relations between crystal structure and external shapes.
SINKANKAS, J. (1964) Mineralogy for Amateurs. (Van Nostrand Reinhold, New York) 290pp.
UBBELHODE, A.R. (1965) The anisotropy of graphite. Endevour 24 (92), 63.
© Copyright John A. Jaszczak
 Back to Jaszczak's home page
Back to Jaszczak's home page Back to Graphite Page
Back to Graphite Page